A Fresh Look at Medicare Drug Price Negotiation Reforms
The recent policy shifts in Medicare’s drug price negotiation program have sparked a lively debate among industry experts, policymakers, and the public alike. Amid the twists and turns of the Inflation Reduction Act (IRA) and a series of recent executive orders, many are left trying to figure a path through the tangled issues of drug pricing reform. In this opinion editorial, we take a neutral stance, break down each new change, and analyze the implications as we get into the legal and economic aspects. Our discussion will use everyday language to help you get into the finer points and subtle details of the current debate.
The Inflation Reduction Act and Its Impact on Medicare
The Inflation Reduction Act of 2022 set in motion a range of reforms that were designed to reduce costs and increase transparency in the Medicare Drug Price Negotiation Program (MDPNP). While the measures were intended to bring down peak spending for Medicare beneficiaries, they were not without their confusing bits and complicated pieces that continue to spark discussion. The act, which originated with a significant push to overhaul outdated drug pricing structures, now stands as a central piece of federal healthcare policy amid an evolving political landscape.
Key Policy Shifts Introduced by the IRA
At its core, the IRA mandated that Medicare negotiate directly with pharmaceutical companies to obtain lower prices for a select group of drugs. For those reading along, the system is undeniably loaded with issues that range from determining which medications receive priority to setting price benchmarks that may be seen as intimidating by industry insiders. Some of the key points include:
- Direct negotiation between Medicare and drug manufacturers for high-cost medications.
- The introduction of a timetable that sees initial negotiations take effect at the turn of the year, starting January 2026.
- Discrepancies in negotiation periods for different types of drugs, which have given rise to what is commonly dubbed the “pill penalty.”
While the intent is to secure savings for taxpayers and patients, critics have raised concerns about the off-putting nature of some of these measures, suggesting that the implementation could lead to unintended consequences in the drug development arena.
Executive Order 14273: Steering Through a New Direction
On April 15, 2025, President Trump issued Executive Order 14273, titled “Lowering Drug Prices by Once Again Putting Americans First.” This directive emerges at a time when the MDPNP is under the microscope, and it represents a clear attempt by the administration to find its way through the existing policies with tweaks meant to outperform previous achievements.
Understanding the Goals of the Executive Order
Executive Order 14273 is a move aimed at reinforcing and refining the Medicare drug price negotiation process. The order is designed to:
- Increase transparency in the negotiation process and ensure that the fine points are open for public and congressional review.
- Set in motion new proposals and guidance on how to roll out the Maximum Fair Price (MFP) for prescription drugs in the upcoming years.
- Prompt inter-agency and congressional cooperation in addressing some of the more nerve-racking parts associated with pricing different classes of drugs.
This order is a clear signal that the current administration is not only satisfied with maintaining the changes brought by the IRA but also is striving for even higher savings targets. With such a directive, the government hopes to secure a 22 percent saving benchmark achieved in the program’s first year while addressing the tangled issues that remain.
Adjusting the MDPNP Timeframes: More Than Just Numbers
One of the most significant discussion points has been the differential treatment between small molecule drugs and biologics. Under the current structure, small molecule drugs are slated for negotiation seven years after FDA approval, with price controls kicking in at year nine, while biologics are given a timeline of 11 and 13 years respectively. This difference has created what many refer to as the “pill penalty.”
The Pill Penalty: A Tangled Issue in Drug Pricing
The “pill penalty” term describes the unintended consequence of having two different timelines for pricing control. As explained by some experts:
- Incentives for Innovation: Critics argue that the shorter timeline for small molecules might discourage investment in this category, potentially leading to a decrease in innovative drugs. They worry that these changes could result in 188 fewer small molecule medicines coming to market, as shown in some policy briefs.
- Impact on Funding: Since funding for small molecule drug research has seen a dramatic downturn, nearly a 70 percent drop since the IRA was implemented, many are concerned that this disparity could exacerbate the underlying issues in pharmaceutical research.
- Alignment Possibilities: In response, bipartisan initiatives, such as Congressman Gregory F. Murphy’s “Ensuring Pathways to Innovative Cures (EPIC) Act,” seek to equalize these negotiation periods by setting uniform eligibility after 11 years for both types. This change is viewed as a key measure in defusing the tension loaded within the current setup.
A table below provides a clear visual contrast between the current timelines:
| Drug Category | Negotiation Eligibility | Price Control Implementation |
|---|---|---|
| Small Molecules | 7 years after FDA approval | 9 years after FDA approval |
| Biologics | 11 years after FDA approval | 13 years after FDA approval |
This table illustrates the fine details and small distinctions that have raised so many questions about fairness and market balance.
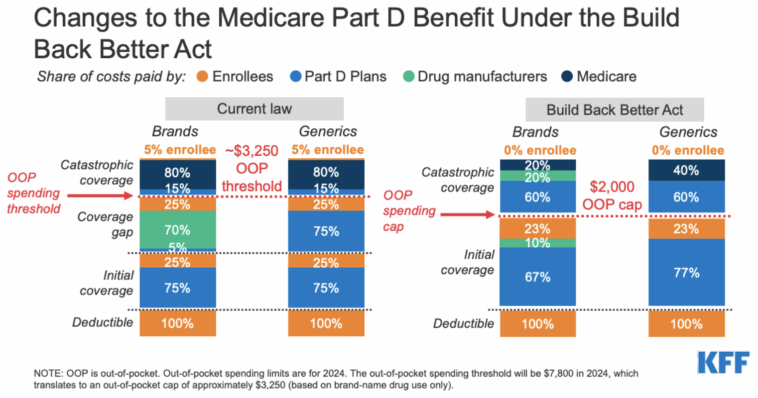
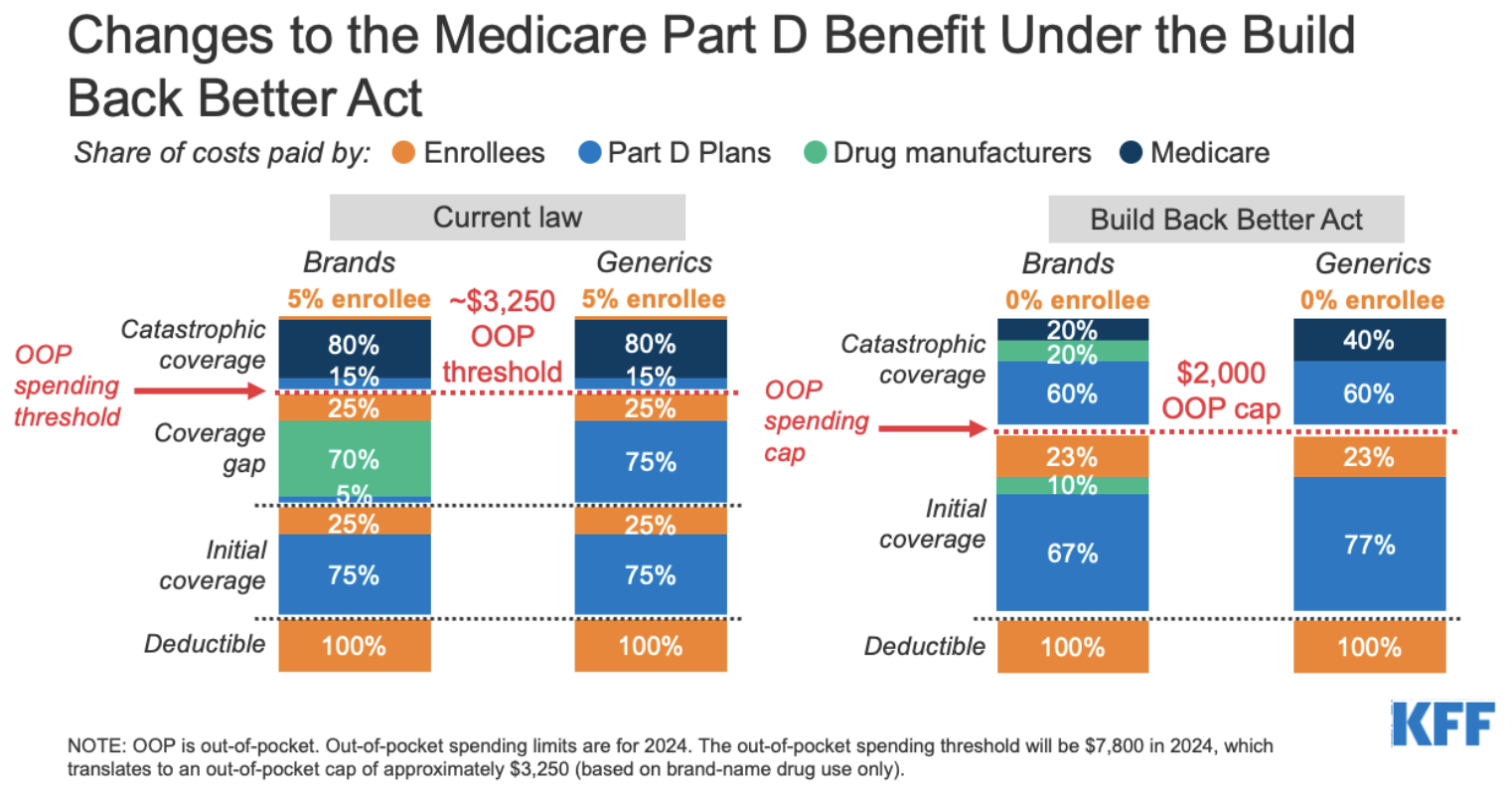
Medicare Part D Redesign: More Than Just a Changing Formulary
Alongside the renewed focus on drug price negotiations, CMS has taken important steps in the redesign of Medicare Part D. The Final CY 2026 Part D Redesign Program Instructions serve as a complementary yet distinct initiative aimed at overhauling how the prescription drug benefit is administered. This set of instructions stands as another key element in the administration’s attempt to get around some of the nerve-racking parts associated with the overall cost structure in Medicare.
What the Part D Redesign Entails
The redesigned instructions bring several changes that affect multiple stakeholders—patients, plan sponsors, and drug manufacturers alike. Some of the key changes include:
- Raised Out-of-Pocket Thresholds: For 2026, the annual out-of-pocket (OOP) threshold is increased slightly, potentially easing the burden on beneficiaries who face high up-front costs.
- Updated Liability Provisions: The instructions clarify and modify the liabilities of enrollees, sponsors, manufacturers, and CMS, aiming to minimize the negative impacts on any single group.
- Selected Drug Subsidy Program: The creation of this program is intended to reduce sponsor liability on the negotiated price for certain selected drugs, thus stabilizing the landscape amid anticipated cost pressures.
- Formulary Substitutions: The program outlines updated criteria for when a drug may be removed from a formulary, an aspect particularly relevant to the earlier negotiation timelines and the subsequent “pill penalty” issue.
CMS has also provided a summary that highlights other critical policies within the instructions, such as the Prescription Drug Plan (PDP) meaningful difference thresholds (moving from 10 to 15 percent), a simplified creditable coverage determination method, and several other small distinctions that are meant to make the entire process more straightforward yet remain loaded with multiple fine points that require close scrutiny.
Evaluating the Overall Impact on Patients and Providers
At its heart, the effort to redesign Part D is driven by the need to offer better value while ensuring that beneficiaries have stable, predictable access to essential drugs. However, as with all sweeping reforms, the implementation is connected with a number of tricky parts:
- Patient Access: Many are debating whether these reforms will truly lower overall costs for patients or simply shift the burden among various parties. The hope is that by streamlining responsibilities and increasing transparency, patients will eventually see more affordable pricing without sacrificing access to important treatments.
- Provider Challenges: Health care professionals and plan sponsors are caught in a balancing act, as they try to steer through the new liability rules while ensuring that drug formularies remain comprehensive. The changes require plan sponsors to get around complex bits of regulation and figure a path that minimizes disruption.
- Market Dynamics: The pharmaceutical industry is watching closely, concerned that these reforms could unintentionally stifle investment in drug research and development. As new payment models are tested, manufacturers are on edge over how quickly their innovative products might be subject to federal price controls.
It is clear that no single stakeholder comes out all winners or all losers in this intricate rework of the Medicare system. Rather, it is a balancing act where every group must manage its way while keeping the overall goal of better patient outcomes and cost savings at the forefront.
Anticipating Future Rulemaking and Its Challenges
One of the key takeaways from these policy shifts is that additional changes are on the horizon. With Secretary Robert F. Kennedy expected to issue further rulemaking seeking public comment by mid-June, interested parties must be ready to take a closer look and provide feedback. This rulemaking process is important for several reasons:
- Public Input: The administration’s openness to feedback is essential for clarifying the fine shades of the policies. Stakeholders—from patient advocacy groups to pharmaceutical associations—have an opportunity to poke around the proposals and share their practical concerns.
- Balancing Innovation With Cost Savings: Any adjustments made in response to the industry’s criticisms or bipartisan proposals (such as those aimed at addressing the “pill penalty”) will need to carefully balance the need for innovation with the demands of cost control.
- Long-Term Stability: Future rulemaking can help cement or modify the current framework, ensuring that short-term savings do not come at the cost of long-term reliability in drug supply, research investment, or patient access.
Key Considerations for Stakeholders
For those ahead in the industry or deeply involved in policy, several key factors should be considered when evaluating future changes:
- Collaborative Engagement: Policy makers and regulators may need to foster a more collaborative environment with diverse stakeholders. This is one way to steer through the intimidating bits of the current reforms, ensuring that the diverse needs of patients, providers, and manufacturers are taken into account.
- Flexibility in Implementation: The rulemaking process should be flexible enough to accommodate adjustments as the effects of current changes become clear. Stakeholders ought to expect some trial and error as they figure a path through this evolving landscape.
- Transparency and Accountability: The public and industry alike demand that the negotiation process remains transparent. Measures to improve clarity can help mitigate confusion and reduce the nerve-racking aspects of the reform, building trust across the board.
Below is an outline summarizing the key steps that need to be taken by both regulators and industry:
| Step | Key Action | Impact on Stakeholders |
|---|---|---|
| 1 | Solicit Broad Public Comment | Ensures diverse viewpoints shape future guidelines. |
| 2 | Reassess Negotiation Timelines | Potentially equalizes treatment for small molecules and biologics. |
| 3 | Clarify Liability Provisions | Helps plan sponsors and manufacturers adjust without overwhelming disruption. |
| 4 | Monitor and Evaluate Early Outcomes | Allows adjustments to be made quickly in response to real-world challenges. |
Putting It All Together: The Road Ahead
In summary, the current landscape of Medicare drug price negotiation reforms is full of confusing bits and tangled issues. The combination of the Inflation Reduction Act, Executive Order 14273, and the redesign of Medicare Part D represents a comprehensive effort to cut down on federal health care spending. While these measures carry significant potential for saving taxpayers money and lowering drug costs for beneficiaries, they are also loaded with challenges that demand careful attention.
The differences in negotiation timelines for small molecules and biologics—the so-called “pill penalty”—continue to be a major point of contention. There is a clear and pressing need for equal treatment in pricing mechanisms to ensure that pharmaceutical innovation does not suffer as a result of overly strict cost controls. In our view, a balanced approach that takes into account the fine shades between immediate savings and long-term benefits is super important for achieving a sustainable healthcare system.
Moreover, the overhaul of Medicare Part D is another arena where federal policy is trying to get around the nerve-racking parts of cost responsibility. Crucial modifications in out-of-pocket thresholds, liability assignments, and formulary management are steps toward balancing patient affordability with industry viability. These changes, while positive on paper, remain subject to the real-world pressure of diverse stakeholder needs.
Looking Beyond the Immediate Reforms
As with any major policy initiative, the journey is far from over. The upcoming rulemaking sessions hold the promise of refining these measures and addressing the subtle parts that have raised concerns among industry veterans and patient advocates alike. For those who are watching this space, it is key to remember:
- Every policy shift introduces its own set of tricky parts—understanding these is crucial to anticipating how new rules will play out in practice.
- Future modifications will likely aim to balance immediate cost savings with long-term innovation, ensuring that drug development remains robust while patient costs decline.
- Transparency remains a must-have element for trust in the system. Clear and open negotiations can smooth over many of the off-putting and nerve-racking details that currently plague the debate.
Stakeholders on all sides—from regulatory bodies to pharmaceutical companies, and from patient groups to healthcare providers—would do well to maintain an open dialogue. This ensures that even as policy evolves, the goals of affordability, access, and innovation continue to receive balanced attention.
Final Thoughts on a Transformative Era in Drug Pricing
This moment in history is pivotal for the future of healthcare in the United States. The current initiatives affecting the Medicare Drug Price Negotiation Program involve a combination of statutory changes and executive directives that seek to cut through some of the overwhelming parts of the system by reengineering key processes. While there are legitimate concerns about the potential for reduced investment in small molecule research or the possibility of higher overall costs shifting to other areas, the active engagement of policymakers and industry experts provides a hopeful indicator that a workable solution is within reach.
As we figure a path through these reforms, one thing remains clear: the system is at a crossroads. Whether you view these changes as a promising breakthrough or a series of nerve-racking adjustments, there is no denying the super important nature of this effort. The administration’s focus on boosting transparency, streamlining negotiations, and ensuring cross-sector collaboration will ultimately determine whether the positive outcomes—lower drug prices and more sustainable healthcare spending—will be realized.
For those interested in keeping up with these developments, it is advisable to stay tuned to public notices, participate in the rulemaking comment periods, and keep an eye on the evolving analyses from legal and economic experts. This is a time when getting into the nitty-gritty of policy details, understanding the subtle distinctions in timelines, and recognizing the potential pitfalls of extreme cost-cutting measures are not just academic exercises—they are essential to ensuring that America’s healthcare landscape becomes better for patients, providers, and innovators alike.
In Conclusion: Charting a Clear Path Forward
In the end, the comprehensive approach to reforming Medicare’s drug price negotiation process represents a bold attempt to address persistent problems in drug pricing. With the IRA setting the stage and subsequent executive actions refining the details, the conversation now shifts to how best to manage the effects of these changes over time. The administration’s goal to secure higher savings while ameliorating the painful bits of the current system is a challenging undertaking, one that clearly requires input from every corner of the healthcare field.
It is our hope that ongoing public discourse, combined with careful rulemaking and active engagement by all interested parties, will result in a framework that fairly balances innovation with cost reduction. While the journey ahead is certainly full of confusing bits and tangled issues, it is also a path filled with the potential for meaningful change. By working together to smooth out the rough twists and turns, we can create a system that is both flexible enough to promote new developments and robust enough to hold down costs for families and taxpayers.
Moving forward, every policymaker, industry leader, and patient advocate must be prepared to figure their way through these challenging reform measures. Whether by refining negotiation timelines, recalibrating liability provisions, or drafting new rules for Part D, every decision along the way will have super important consequences for the future. In this transformative era, getting into the fine points and managing your way through each legal and regulatory update is not merely an academic exercise—it is a critical responsibility to ensure that the aim of providing affordable, high-quality healthcare remains within reach.
As we take a closer look at the ongoing developments, one thing remains abundantly clear: the conversation about drug pricing is far from over. The initiatives of today are the stepping stones to tomorrow’s policies, and every stakeholder has a role to play in shaping a system that prioritizes the well-being of all Americans. It is only through careful analysis, continuous dialogue, and a willingness to adjust course when the path becomes too laden with tricky parts that we will eventually arrive at a fair and effective solution.
In conclusion, whether you are a policy wonk, a healthcare provider, or simply a citizen trying to make sense of these reforms, there is no denying that the current changes represent a significant, and indeed transformative, shift in American healthcare. With persistent monitoring, open debate, and a collaborative spirit, we can hope that these changes will pave the way for a system that is as innovative as it is affordable—a system that not only saves money but also preserves the essential drive for innovation in drug development. The journey may be challenging and, at times, overwhelming, but it is a journey worth taking for the benefit of present and future generations.
Originally Post From https://natlawreview.com/article/medicare-drug-price-negotiation-program-inflation-reduction-act-pill-penalty-and
Read more about this topic at
Lowering Drug Prices by Once Again Putting Americans First
Fact Sheet: President Donald J. Trump Announces Actions …